Role of TACE/ADAM17 in TNF-α-driven sympathetic activation in heart failure (HF)
Role of TACE/ADAM17 in TNF-α-driven sympathetic activation in heart failure (HF)
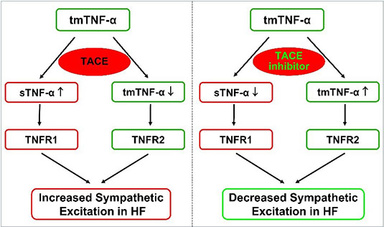
Tumor necrosis factor-α (TNF-α), one of the key pro-inflammatory cytokines, plays an important role in the pathogenesis of HF. In patients with HF, increased circulating cytokine TNF-α is closely correlated to the severity of HF and are sufficient to cause cardiac dysfunction. The active soluble form of TNF-α (sTNF- α) is initially produced as a membrane bound protein. sTNF-α must be cleaved from transmembrane TNF-α (tmTNF-α) by TNF-α converting enzyme (TACE), a disintegrin and metalloprotease 17 (ADAM17), to become the fully functional mediator. Subsequently, sTNF-α binds predominantly to the TNF receptor 1(TNFR1) to elicit pro-inflammatory and toxic responses. The remaining tmTNF- binds predominantly to the TNF receptor 2 (TNFR2) which has been demonstrated to have an anti-inflammatory and protective effect in a variety of pathophysiological states. Both sTNF-α and tmTNF-α exhibit biological functions, but with generally opposing roles. We propose that the balance between these two forms of TNF- and their individually predominant receptors in critical brain regions is an important determinant of the level of neurohumoral excitation in HF. We are currently investigating whether central interventions inhibiting TACE activity lessen sympathetic activation and improve cardiac function in myocardial infarction-induced heart failure.
Interleukin-17 and neuroinflammation in cardiovascular diseases
Interleukin-17 and neuroinflammation in cardiovascular diseases
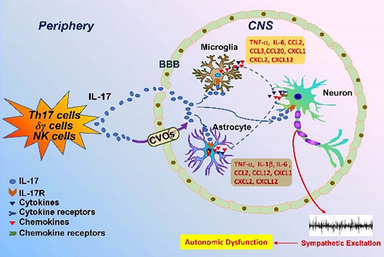
The highly pathogenic pro-inflammatory cytokine Interleukin-17 (IL-17, also known as IL-17A) is a documented target in chronic inflammation. IL-17A is placed in a unique position because of its property in connecting immune responses and tissue inflammation. IL-17A is the founding member of IL-17 cytokine family, which is secreted mainly by Th17 cells and γδ T cells. Over the years, IL-17 has been identified as a critical inflammatory regulator to promote inflammatory states in cardiovascular diseases by amplifying the expression of various inflammatory mediators including cytokines and chemokines. There is evidence indicating that IL-17A can impair the integrity of blood brain barrier and activate astrocytes and microglia in the brain. Its receptors, IL-17RA and IL-17RC, are abundantly expressed in CNS. Moreover, IL-17A has a synergistic effect with other inflammatory cytokines to magnify its consequences. We hypothesize that IL-17A acts centrally to influence sympathetic drive and cardiovascular dynamics. We further investigate the potential role of IL-17A in sympathetic activation in chronic inflammatory disease settings like heart failure and hypertension.
Central inflammatory mechanisms underlying neurohumoral activation in heart failure
Central inflammatory mechanisms underlying neurohumoral activation in heart failure
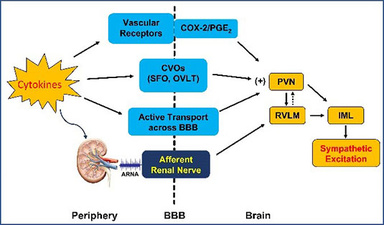
Inflammation has been implicated in the pathophysiology of cardiovascular diseases like hypertension and heart failure. We investigate the inflammatory mechanisms of sympathetic excitation in two aspects: 1). To identify the neural pathways that mediate peripheral inflammation-induced sympathetic excitation. Since cytokines are too large to cross the blood brain barrier (BBB), how peripheral inflammatory mediators access the brain to activate autonomic nervous system remains a mystery. Our previous study found that blood-borne cytokines can access the brain via circumventricular organs (CVOs) like SFO, a brain region lacking the BBB (Hypertension. 2013;62(1):118-25 & Hypertension. 2015, 65(5):1126-33). Additionally, the circulating cytokines could stimulate cyclooxygenase-2 (COX-2) activity in perivascular macrophages of brain vessels to activate COX-2/PGE2 axis and promote neurohumoral activation (Hypertension. 2010;55(3):652-9). Most recently, our study discovered that afferent renal nerve may play an important role in mediating peripheral inflammation-induced sympathetic excitation. The afferent renal nerve is composed of chemoreceptor and mechanoreceptor fibers, and delivers peripheral signals to the central nervous system. Further study will determine whether afferent renal nerve-mediated inflammatory signals contribute to the progression of heart failure; 2) To examine how inflammatory mediators act within the brain to induce neuronal excitability and synaptic plasticity to advance sympathetic nervous system activation, neuroendocrine secretion and hemodynamic changes in heart failure and hypertension, and what are the intracellular signaling pathways and downstream excitatory products in response to the stimulation by proinflammatory cytokines.
Global analysis of transcriptional remodeling in heart failure
Global analysis of transcriptional remodeling in heart failure
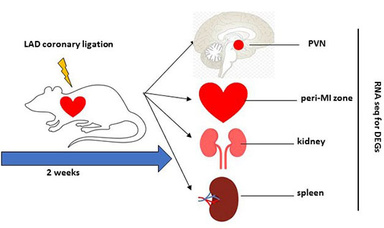
The reduced cardiac output in heart failure (HF) leads to insufficient blood supply and tissue injury to the organs, which evokes numerous neural and humoral compensatory responses to maintain normal blood pressure and the performance of the heart. The compensatory mechanisms in the development of heart failure may result in transcriptional modification or epigenetic changes in the chromatins of individual functional organs. However, the alternations of gene expression affected by left ventricle dysfunction in HF is not completely understood. One of our research projects currently seeks to determine the transcription landscape remodeling and adaptive epigenetic variations in HF. To gain insights into global transcriptional changes, we employ an unbiased whole-genome RNA sequencing approach to interrogate the transcriptional differences in multiple organs including heart, brain, kidney, and spleen in the HF vs. sham animals. Preliminary data we obtained support the concept that heart failure is a multi-organ syndrome. Principle component analysis (PCA) indicates that there are a variety of upregulated or downregulated differentially expressed genes (DEGs) in these organs in HF compared with sham animals. Particularly, we found multiple inflammation-related pathways that are suppressed in spleens of HF rats, which most likely represents a rapid deployment event of spleen-derived leukocytes in responses to the homeostatic alternations in HF. These immune cells such as T cells and monocytes may play an important role in the development of HF. Our initial findings from the global analysis of transcriptional remodeling suggest a perplexing regulatory network that contributes to the inflammatory and autonomic regulation in HF. Further study will determine the functional significance of these DEGs identified in these organs. We will also determine whether there is a sex difference on the transcriptional remodeling and adaptive epigenetic changes in HF.
Development of drug delivery approaches in treating heart failure
Development of drug delivery approaches in treating heart failure
Nanomedicine has been widely employed as a site-specific and target-oriented delivery strategy in treating cardiovascular diseases. Currently, the synthetic biomimetic nanoparticles and extracellular vesicles (EVs) are two major types of nanoparticles that are rapidly developed to serve as a therapeutic tactic or an effective drug delivery system to the treatment of heart failure. It is well-established that heart failure is characterized by activated autonomic nervous system. However, central intervention targeting the brain to ameliorate the sympathetic excitation by systemically administered therapeutic agents is a major challenge due to the protection by blood brain barrier. The nanotechnology with the potential to cross the blood-brain barrier is a promising means that can facilitate the delivery of the drugs to the brain and thus mitigate the neurohumoral activation in the setting of heart failure. Our laboratory is working on two formations of nanoparticles and hope to develop a novel therapeutic and/or nanodelivery approach that may offer substantial benefits in treating this devastating disease.
Preparation of biomimetic nanoparticles: To target the inflamed tissues, biomimetic nanoparticles can be rationally designed to mimic the natural processes of inflammatory response which is often neglected in the traditional design of nanomedicine. As illustrated by the schematic, biomimetic nanoparticles are prepared using immune cells that are isolated from bone marrow and stimulated by LPS. The plasma membrane from LPS-activated cells is collected through the processes of centrifugation and extrusion. The designed liposomes containing therapeutic agents are constructed by film dispersion method. Biomimetic nanoparticles are made by coating the LPS-stimulated cell membrane onto the designed-liposomes. This formation of nanomedicine combines the advantages of both synthetic nanoscale materials and the natural functions of coated immune proteins to serve as an immune-facilitated drug delivery means. With proper coating, biomimetic nanoparticles are expected to carry the drugs to the specific targeted site to improve the therapeutic effects in treating heart failure.

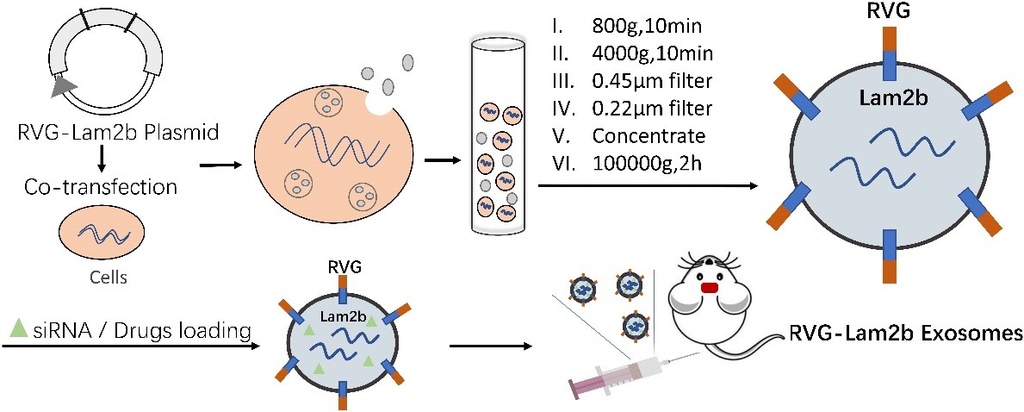
Preparation of RVG-exosomes based nanoparticles: Exosomes, a subtype of bilayer EVs released from almost all cells, are well-documented as the key messengers for intercellular communication including the glia-neuron interaction in the central nervous system. The EVs that are composed of proteins, lipids, soluble small molecules, and nucleic acids play an important role in regulating physiological or pathological processes because of the capacity to transfer the content, or cargo of EVs from producer cells to the targeted cells in distinct organs. More important, EVs released from cells in the periphery can cross the blood-brain barrier effectively and thus deliver the therapeutic agents including proteins, chemicals, and nucleic acid into the brain. The Schematic illustrates the preparation of extracellular vesicle-based nanoparticles. The rabies virus glycoprotein (RVG) conjugated EVs are constructed via fusing RVG to lysosomal-associated membrane glycoprotein 2b protein (Lamp2b-RVG) on the EVs surface. The cells of interest are co-transfected with pcDNA3.1(−)-RVG-Lamp2b plasmids. The RVG-Lam2b EVs are collected from the supernatant of culture media and isolated through several processing steps. The RVG peptides on the surface facilitate the modified RVG-Lam2b EVs loaded with various therapeutic agents to gain access the brain. This designed EVs would be an effective tool to transport the therapeutic drugs such as chemicals, proteins or nucleic acids to the central nervous system.